PROTEOSTAT® Aggresome detection kit
蛋白聚集小体检测试剂盒
- 产品特性
- 相关资料
- Q&A
- 参考文献
蛋白聚集小体检测试剂盒![]()
PROTEOSTAT® Aggresome detection kit
◆原理
聚集小体(Aggresome)是由一群不正常堆叠在一起的蛋白质所形成的包涵体(Inclusion Bodies)。聚集小体的出现往往也表明细胞正处于某种应激状态,比如高温、病毒感染、活性氧自由基攻击等。聚集体通常是响应细胞应激而形成,并通过分离错误折叠的蛋白质提供细胞保护机制,最终导致它们被自噬清除。此外,蛋白质聚集体的形成是多种人类疾病的标志,例如阿尔茨海默氏症、帕金森氏症、肌萎缩侧索硬化症或酒精性肝病。
PROTEOSTAT® Aggresome detection kit中包含的 PROTEOSTAT® 是一种分子转子染料,在溶液中沿着单个中心键的自由分子内旋转可防止荧光产生。当它特异地插入到错误折叠和聚集的蛋白中的交叉β-sheet四级结构中,旋转受到抑制并导致发出强烈的荧光。
PROTEOSTAT® 聚集小体检测试剂盒提供了一种快速、特异、定量的方法来标记细胞中的聚集小体,且无需进行人工蛋白定点突变。PROTEOSTAT® 染料已在广泛的条件下对多种小分子调节剂进行验证,适用于具有治疗价值的化学物的筛选。此外,本试剂盒还适用于多重免疫荧光,以在自噬和聚集体形成的背景下研究您的目标分子。
◆试剂盒组成
|
组分 |
包装 |
|
PROTEOSTAT® Aggresome Detection Reagent |
10 μL |
|
Hoechst 33342 Nuclear Stain |
50 μL |
|
Proteasome Inhibitor (MG-132) |
120 nM |
|
10×Assay Buffer |
25 mL |
◆特点
● 提供基于细胞的灵敏的药物反应性测定,以在真实的细胞环境中识别与神经退行性疾病相关的抑制剂
● 可靠且简单的检测,不需要非生理性蛋白质突变或基因工程细胞系
● 作用条件广泛,适用于筛选具有潜在治疗价值的小分子化合物
● 已进行优化以兼容免疫染色
● 可通过流式细胞术轻松量化聚集体和相关包涵体
● 可用于神经退行性疾病、肝病、毒理学研究等的研究
◆案例・应用
■ 应用实例 1:流式细胞术检测聚集小体
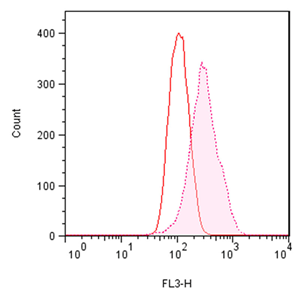
流式细胞术的分析:Jurkat细胞在37°C下用 0.2% DMSO对照或用5 µM MG-132诱导过夜。处理后,将细胞固定并与PROTEOSTAT® 染料一起孵育,无需洗涤即通过流式细胞术在FL3通道中使用488 nm激光进行分析。在 MG-132处理的细胞中,红色荧光信号增加约3倍。所描述的测定允许评估蛋白质聚集的影响。
■ 应用实例 2:PROTEOSTAT® 染料与荧光染料的应用
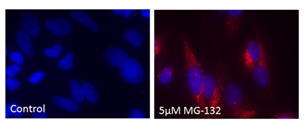
荧光显微镜:含有蛋白聚集体的HeLa细胞,用 5 µM MG-132蛋白酶体抑制剂(右)处理12小时,通过 PROTEOSTAT®(红色)检测并用Hoechst 33342复染。左侧的为未经处理的对照。
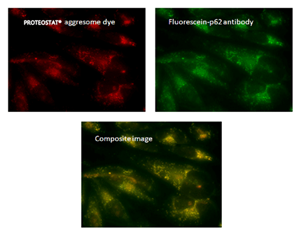
荧光显微镜:含有蛋白聚集体的HeLa细胞,用 5μM MG-132蛋白酶体抑制剂处理12小时,通过PROTEOSTAT® 聚集体染料(左上)检测,显示与荧光素-p62抗体(右上)和复合图像(中下)的共定位
■ 应用实例 3:不同搅拌速率对蛋白聚集的影响
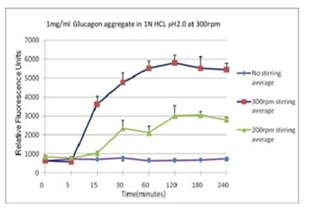
使用本产品检测后,可知搅拌速度越快,蛋白聚集程度越大。
■ 应用实例 4:蛋氨酸氧化可抑制蛋白聚集
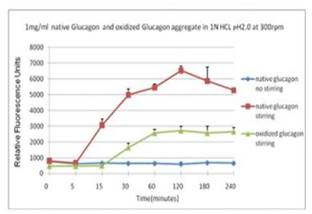
蓝色曲线为对照,没有搅拌;在搅拌速度在300 rpm下, 绿色曲线与红色曲线分别表示蛋氨酸被氧化后的蛋白聚集情况。
使用本产品检测后,可知蛋氨酸被氧化后可抑制蛋白聚集。
■ 应用实例 5 :内质网应激相关细胞自噬产生蛋白聚集小体
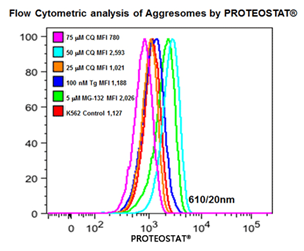
分别25,50,75 mmCQ,100 nM的thapsigargin(TG)或5mM的MG-132处理K562细胞24小时。然后在温室下用PROTEOSTAT® (1:10,000)固定并透化细胞30min。接着在BD流式细胞仪LSR II的蓝色610/20 nm的通道分析细胞(30,000)。
图片来源:Courtesy of the Flow Cytometry Core Facility, Blizard Institute, Queen Mary University of London, London, UK.
■ 应用实例 6
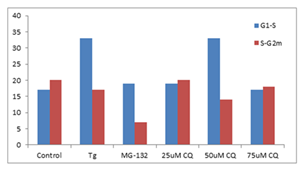
K562 细胞分别使用Thapsigargin (Tg, 0.1 µM)、25、50 和 75 µM 氯喹 (CQ) 用试剂处理以诱导内质网应激和不完全自噬 24 h。使用蛋白酶体抑制剂 MG-132 (5 µM) 作为阳性对照处理24 h。将沉淀的细胞固定并透化。然后,根据制造商的说明,使用 300 µL PROTEOSTAT® 和 1 µg/mL DAPI(用于细胞周期测定)在室温下标记细胞30min。确定每个处理的S期的 Aggresome 倾向因子 (APF) 高于G1和高于S期的G2m的相对增加,并与对照值进行比较。与对照组相比,ER 应激诱导剂、Tg 和 50 µM CQ在S期比G1更能引起蛋白聚集体上调,但蛋白酶体抑制剂 MG-132 和 50 µM CQ 在 G2m 期比S期更显著地下调聚集体。
图片来源:Courtesy of the Flow Cytometry Core Facility, Blizard Institute, Queen Mary University of London, London, UK.
PROTEOSTAT® Aggresome Detection Kit
PROTEOSTAT® 蛋白聚集检测试剂盒 Q&A
1.Q: PROTEOSTAT® 热稳定检测试剂盒(ENZ-51027),蛋白聚集检测试剂盒(ENZ-51023)
1.Q: 和PROTEOSTAT® 蛋白聚集检测试剂盒(ENZ-51035)的区别?
1.A: 1)PROTEOSTAT®染料属于分子轮子染料(molecular rotor dye)。在溶液状态不发荧光(由于在中心的碳-碳单键周围的自由转动,
分离了探针的不同芳香部分)。由于分子轮子染料接触到蛋白聚集体后,会封闭进入原纤维,形成β折叠,具有高强度荧光。类似于
染核酸碱基的EB 一样。
1.A: 2)PROTEOSTAT® 热稳定检测试剂盒(ENZ-51027)用于直接监测热诱导蛋白变性引起的蛋白聚集,不检测蛋白非折叠而暴露的疏
水部分。该试剂盒用于检测蛋白缓冲液,其他添加成分对蛋白稳定性的影响,在特定条件下多少温度时蛋白会聚集。
1. A 3)蛋白聚集检测试剂盒(ENZ-51023)用于检测液体状蛋白或者多肽形成聚集的情况。可用于确认蛋白保存的最优形态,筛选促进或
者抑制蛋白聚集的试剂,检测分子伴侣的活性。与标准品或者已知浓度样品一起使用,可定量检测蛋白聚集。
1. A 4)PROTEOSTAT® 蛋白聚集检测试剂盒(ENZ-51035)检测变性蛋白,包括活细胞内聚集体或者聚集体样包涵体。PROTEOSTAT®
蛋白聚集检测染料在聚集小体形成时,接触到聚集蛋白时会变的更明亮,可用流式或者荧光显微镜检测。
2.Q: 使用ENZ-51035 时,是否可以终止并在在40℃过夜保存,第二天继续实验?
A: 建议可以在加染料前终止。荧光强度在液体中会成倍降低。而且染料与蛋白长时间孵育后,会影响蛋白聚集。
3.Q: 阳性对照染色不好。
A: 染料没有避光保存。染色时候没有避光,需要染色后立即检测。聚集蛋白不是液体。延长离心会使聚集蛋白沉淀。不要离心聚集蛋
白(样品或者对照)。
4.Q: 蛋白信号饱和。
A: 蛋白样品的浓度很高。用1×Assay Buffer 稀释样品。
5.Q: 阴性对照观察到高荧光强度。
A: 样品含有干扰物质。该试剂可以与常规使用的缓冲液(PBS,Tris,HEPES)和赋形剂(海藻糖和蔗糖)使用,不过不要使用高浓度的吐温
20(比如:0.2%)
6.Q: 蛋白与PROTEOSTAT® 检测试剂结合是否是可逆的?
A: PROTEOSTAT® 检测试剂与聚集蛋白的相互作用是非供价结合。所以,理论上是可逆的。但是我们没有尝试将染料从样品中去除。
7.Q: 在药物应激检测中,细胞是否可以经胰酶消化后去检测细胞中的蛋白聚集?
A: 胰酶可以与PROTEOSTAT® 聚集检测试剂盒配套使用。细胞消化后,表面活性剂加入给细胞打孔。更大的聚集体仍然是不溶的,可
以通过离心沉淀。小的聚集体通过超滤分离。表面活性剂,膜和可溶蛋白可洗掉,PROTEOSTAT® 检测试剂可用于残留蛋白。
8.Q: 在研究细胞蛋白聚集时,如何设置阳性对照?
A: MG 132 处理的细胞可作为阳性对照。
9.Q: 细胞裂解液是否可用PROTEOSTAT® 蛋白聚集检测试剂检测?
A: 检测细胞裂解液是有挑战的,表面活性剂会引起高背景。可以过滤去除表面活性剂。更大的聚集体仍然是不可溶的,可以通过
离心沉淀。小的聚集体通过超滤分离。表面活性剂,膜和可溶蛋白可洗掉,PROTEOSTAT® 检测试剂可用于残留蛋白。
10.Q: PROTEOSTAT® 染料是否可以检测~300kDa 的蛋白二聚体?
A: PROTEOSTAT® 可以检测从单体到二聚体转变的蛋白,结合分子筛层析法。但是信号会变的更强烈,因为聚集体更大。
11.Q: 如果样品稀释到低浓度(比如0.5mg/mL)是否会改变聚集蛋白的百分比含量?
A: 聚集蛋白的百分比(聚集蛋白占总蛋白的比例)将保持不变。聚集蛋白的浓度会降低,信号也会减弱。
A: 如果用未聚集蛋白稀释样品,聚体蛋白的百分比会变化。
12.Q: PROTEOSTAT® 试剂盒是否可用于研究原核细胞样品的蛋白聚集?
A: 如果膜部分去除的话,是可以检测原核细胞的。如果膜没有分离,染料会聚集在膜上,引起高背景值。
13.Q: PROTEOSTAT® 是否可检测革兰氏阴性菌的蛋白聚集?
A: 革兰氏阴性菌外层膜的脂多糖会影响。操作手册中提到的打孔缓冲液将移除大多数细菌的内层膜,破坏革兰氏阴性菌的外层膜。所以
可以观察革兰氏阴性菌的聚集和包涵体。
14.Q: 当其他蛋白共染时,如何优化减少FITC 发射过滤装置的信号?
A: 染料的激发和发射光谱见操作手册P8。光谱显示会与FITC 有些重叠。FITC 可以与该染料一起使用,用488nm激发波长和515nm 发
射波长。如果FITC 有荧光的话就会起作用。否则,PROTEOSTAT® 染料会有溢出。建立设立一个不带有FITC 标记的抗体作为对照。
另外,可用Cy5 或者Coumarin 标记的抗体解决这个问题。
|
1. |
A fast and specific method to screen for intracellular amyloid inhibitors using bacterial model systems: S. Navarro, et al.; Eur. J. Med. Chem. (2015), Application(s): Confocal microscopy, Abstract; |
|
2. |
Amyloidogenic lysozymes accumulate in the endoplasmic reticulum accompanied by the augmentation of ER stress signals: Y. Kamada, et al.; Biochim. Biophys. Acta 1850, 1107 (2015), Application(s): Microscopy, Abstract; Conophylline protects cells in cellular models of neurodegenerative diseases by inducing mammalian target of rapamycin (mTOR)-independent autophagy: Y. Sasazawa, et al.; J. Biol. Chem. 290, 6168 (2015), Abstract; |
|
3. |
Decreased proteasomal function accelerates cigarette smoke-induced pulmonary emphysema in mice: Y. Yamada, et al.; Lab. Invest. 95, 625 (2015), Application(s):Aggresome detection by fluorescence microscopy in fibroblasts, Abstract; |
|
4. |
Defective autophagy is a key feature of cerebral cavernous malformations: S. Marchi, et al.; EMBO Mol. Med. 7, 1403 (2015), Application(s): Aggresome detection in aggregated proteins and aggresome‐like inclusion bodies in fixed and permeabilized samples,Abstract; Full Text |
|
5. |
Fibril growth and seeding capacity play key roles in α-synuclein-mediated apoptotic cell death: A.L. Mahul-Mellier, et al.; Cell Death Differ. 22, 2107 (2015), Abstract; |
|
6. |
In vitro administration of gold nanoparticles functionalized with MUC-1 protein fragment generates anticancer vaccine response via macrophage activation and polarization mechanism: T. Mocan, et al.; J. Cancer 6, 583 (2015), Application(s): Aggresome detection by fluorescence microscopy in peritoneal macrophages, Abstract; Full Text |
|
7. |
Intensified autophagy compromises the efficacy of radiotherapy against prostate cancer: M.I. Koukourakis, et al.; Biochem. Biophys. Res. Commun. 461, 268 (2015),Application(s): Fluorescence microscopy , Abstract; Mevalonate pathway regulates cell size homeostasis and proteostasis through autophagy: T.P. Miettinen, et al.; Cell Rep. 13, 2610 (2015), Application(s): Flow cytometry analysis of protein aggregation using Jurkat, U2OS, Kc167 and HUVEC cells, Abstract; |
|
8. |
MiR-29b replacement inhibits proteasomes and disrupts aggresome+autophagosome formation to enhance the antimyeloma benefit of bortezomib: S. Jagannathan, et al.; Leukemia 29, 727 (2015), Application(s): Detection of protein aggregates by fluorescence microscopy in multiple myeloma cell lines, Abstract; Full Text |
|
9. |
Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma: M.A. Abdel Malek, et al.; Oncotarget 6, 3098 (2015), Application(s): Confocal Microscopy, Abstract; Full Text |
|
10. |
Monitoring of dipeptidyl peptidase-IV (DPP-IV) activity in patients with mucopolysaccharidoses types I and II on enzyme replacement therapy – Results of a pilot study: K. Hetmanczyk, et al.; Clin. Biochem. (2015), Application(s): Plasma DPP-IV enzyme assay, Abstract; |
|
11. |
Pressure overload-induced cardiac dysfunction in aged male adiponectin knockout mice is associated with autophagy deficiency: J.W. Jahng, et al.; Endocrinology 156, 1667 (2015),Abstract; Protein kinase C-dependent growth-associated protein 43 phosphorylation regulates gephyrin aggregation at developing GABAergic synapses: C.Y. Wang, et al.; Mol. Cell. Biol.35, 1712 (2015), Abstract; Schwann cells contribute to neurodegeneration in transthyretin amyloidosis: T. Murakami, et al.; J. Neurochem. 134, 66 (2015), Abstract; |
|
12. |
Cationic polystyrene nanospheres induce autophagic cell death through the induction of endoplasmic reticulum stress: H.W. Chiu, et al.; Nanoscale 7, 736 (2014), Abstract; Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen: S.M. Usmani, et al.; Nat. Commun. 5, 3508 (2014), Application: Amyloid detection in semen,Abstract; |
|
13. |
Distinct patterns of HSP30 and HSP70 degradation in Xenopus laevis A6 cells recovering from thermal stress: S. Khan, et al.; Comp. Biochem. Physiol. A Mol. Integr. Physiol. 168, 1 (2014), Application(s): Detection of aggresomes in Xenopus laevis cells using fluorescence microscopy, Abstract; Dynein function and protein clearance changes in tumor cells induced by a kunitz-type molecule, amblyomin-x: M.T. Pacheco, et al.; PLoS One 9, e111907 (2014), Application(s):Detection of aggresomes by flow cytometry, Abstract; Full Text |
|
14. |
Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication: A. Flierl, et al.; PLoS One 9, e112413 (2014), Application(s):Detection of protein aggregates by fluorescence microscopy and flow cytometry in neuronal precursor cells, Abstract; Full Text |
|
15. |
Human stefin B role in cell's response to misfolded proteins and autophagy: M. Polajnar, et al.; PLoS One 9, e102500 (2014), Application(s): Detection of protein aggregates in primary astrocytes, Abstract; Full Text |
|
16. |
Novel estradiol analogue induces apoptosis and autophagy in esophageal carcinoma cells: E. Wolmarans, et al.; Cell Mol. Biol. Lett. 19, 98 (2014), Abstract; |
|
17. |
Preconditioning stimulus of proteasome inhibitor enhances aggresome formation and autophagy in differentiated SH-SY5Y cells: Y. Bang, et al.; Neurosci. Lett. 566, 263 (2014),Abstract; |
|
18. |
Protein deubiquitination during oocyte maturation influences sperm function during fertilisation, antipolyspermy defense and embryo development: Y.J. Yi, et al.; Reprod. Fertil. Dev. (2014), Application(s): Detection of protein aggregates in oocytes, Abstract; |
|
19. |
Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination: C.E. Kennedy, et al.; Mol. Reprod. Dev. 81, 436 (2014), Abstract; Serine/threonine kinase 16 and MAL2 regulate constitutive secretion of soluble cargo in hepatic cells: J.G. In, et al.; Biochem. J. 463, 201 (2014), Abstract; |
|
20. |
SGTA regulates the cytosolic quality control of hydrophobic substrates: L. Wunderley, et al.; J. Cell. Sci. 127, 4728 (2014), Application(s): Dual staining with ProteoStat® dye, Abstract;Full Text |
|
21. |
T he small heat shock protein B8 (HSPB8) confers resistance to bortezomib by promoting autophagic removal of misfolded proteins in multiple myeloma cells: M. Hamouda, et al.; Oncotarget 5, 6252 (2014), Application(s): Analysis of velcade resistant multiple myeloma human cells by WB, Assay, Abstract; Full Text |
|
22. |
Aldosterone and angiotensin II induce protein aggregation in renal proximal tubules: M.U. Cheema, et al.; Physiol. Rep. 1, e00064 (2013), Application(s): Labeling of kidney homogenates, labeled particles sorted by flow cytometry and identification by LC-MS/MS ,Abstract; Full Text |
|
23. |
Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death: P. Magnaghi, et al.; Nat. Chem. Biol. 9, 548 (2013), Application(s): Detection of aggresomes in human colon carcinoma HCT116 cells using fluorescence microscopy, Abstract; |
|
24. |
Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner: Y. Nakajima, et al.; Int. J. Mol. Sci. 14, 7771 (2013),Application(s): Detection of aggresomes using fluorescence microscopy, Abstract; Full Text |
|
25. |
In vitro changes in mitochondrial potential, aggresome formation and caspase activity by a novel 17-β-estradiol analogue in breast adenocarcinoma cells: D.S. Nkandeu, et al.; Cell. Biochem. Funct. 31, 566 (2013), Abstract; Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons: H. Liu, et al.; Neurotox. Res. 24, 191 (2013), Abstract; |
|
26. |
Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells: S. Moriya, et al.; Int. J. Oncol.42, 1541 (2013), Application(s): Detection of aggresomes using flow cytometry, Abstract;Full Text |
|
27. |
Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2: Y. Maejima, et al.; Nat. Med. 19, 1478 (2013), Application(s): Detection of aggresomes in mouse heart sections using fluorescence microscopy, Abstract; Full Text |
|
28. |
N-terminally truncated forms of human cathepsin F accumulate in aggresome-like inclusions: B. Jeric, et al.; Biochim. Biophys. Acta 1833, 2254 (2013), Application(s):Detection of aggresomes using fluorescence microscopy, Abstract; |
|
29. |
The ubiquitin proteasome system regulates the stability and activity of the glucose sensor glucokinase in pancreatic beta cells: A. Hofmeister-Brix, et al.; Biochem. J. 456, 173 (2013),Abstract; Full Text |
|
30. |
VCP Phosphorylation-Dependent Interaction Partners Prevent Apoptosis in Helicobacter pylori-Infected Gastric Epithelial Cells: C.C. Yu, et al.; PLoS One 8, e55724 (2013),Application(s): Aggresome detection in AGS human gastric epithelial cells, Abstract; Full Text |
|
31. |
Zerumbone, an electrophilic sesquiterpene, induces cellular proteo-stress leading to activation of ubiquitin-proteasome system and autophagy: K. Ohnishi, et al.; BBRC 430, 616 (2013), Application(s): Aggresome detection in mouse hepatocytes, Abstract; |
|
32. |
Autophagy in idiopathic pulmonary fibrosis: A.S. Patel, et al.; PLoS One 7, e41394 (2012),Application(s): Detection of aggresomes in lung tissue sections using fluorescence microscopy, Abstract; Full Text |
|
33. |
Decreased proteasomal activity causes age-related phenotypes and promotes the development of metabolic abnormalities: U. Tomaru, et al.; Am. J. Pathol. 180, 963 (2012),Abstract; |
|
34. |
Mutations in the area composita protein αT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy: J. van Hengel, et al.; Eur. Heart J. 34, 201 (2012),Abstract; |
|
35. |
Quantitative analysis of α-synuclein solubility in living cells using split GFP complementation: A. Kothawala, et al.; PLoS One 7, e43505 (2012), Application(s):Aggresome detection in HeLa cells, Abstract; Full Text |
|
36. |
Multiple aggregates and aggresomes of C-terminal truncated human αA-crystallins in mammalian cells and protection by αB-crystallin: I. Raju, et al.; PLoS One 6, e19876 (2011), Application(s): Aggresome detection in HeLa cells, Abstract; Full Text |
|
37. |
Novel Cell- and Tissue-Based Assays for Detecting Misfolded and Aggregated Protein Accumulation Within Aggresomes and Inclusion Bodies: D. Shen, et al.; Cell Biochem. Biophys. 60, 173 (2011), Abstract; Full Text |
|
38. |
Inhibitors of protein aggregation and toxicity: H. Amijee, et al.; Biochem. Soc. Trans. 37, 692 (2009), Abstract; Full Text |
|
39. |
Autophagy-mediated clearance of aggresomes is not a universal phenomenon: E. Wong, et al.; Hum. Mol. Genet. 17, 2570 (2008), Abstract; Full Text |
|
40. |
Chemical and biological approaches synergize to ameliorate protein-folding diseases: T.W. Mu, et al. ; Cell 134, 769 (2008), Abstract; Full Text |
|
41. |
Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells: Y. Murakawa, et al.; Cancer Res. 67, 8536 (2007), Abstract; |
|
42. |
p62SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death: G. Bjørkøy, et al.; J.Cell Biol. 171, 603 (2005), Full Text |
|
43. |
Therapeutic effects of cystamine in a murine model of Huntington's disease: A. Dedeoglu, et al.; J. Neurosci. 22, 8942 (2002), Full Text |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 | 备注 |
| ENZ-51035-K100 | PROTEOSTAT® Aggresome detection kit PROTEOSTAT®蛋白聚集小体检测试剂盒 |
100 tests | – | – |
| ENZ-51035-0025 | PROTEOSTAT® Aggresome detection kit PROTEOSTAT® 蛋白聚集小体检测试剂盒 |
25 tests | – | – |
| 免责声明 |
|
1. 本公司密切关注本网站发布的内容,但不保证发布内容的准确性、完整性、可靠性和最新性等。 2. 本公司不保证使用本网站期间不会出现故障或计算机病毒污染的风险。 3. 无论何种原因,使用本网站时给用户或第三方造成的任何不利或损害,本公司概不负责。此外,对于用户与其他用户或第三方之间因本网站发生的任何交易、通讯 3. 或纠纷,本公司概不负责。 4. 本网站可提供的所有产品和服务均不得用于人体或动物的临床诊断或治疗,仅可用于科研等非医疗目的。如任何用户将本网站提供的产品和服务用于临床诊断或治 4. 疗,以及其他特定的用途或行为,本公司概不保证其安全性和有效性,并且不负任何相关的法律责任。 |
